As the healthcare industry continues to pursue safety, comfort, and exceptional performance, medical pressure-sensitive adhesives (Medical PSA) play an increasingly critical role across many medical applications. One key advantage of medical-grade silicone is its ability to meet the demanding requirements of medical PSAs, offering superior biocompatibility, durability, and customization for healthcare applications. Why is it necessary to add MQ organosilicon resin to medical pressure-sensitive adhesives?
Silicone materials—particularly those based on medical-grade silicone compounds—have emerged as a game-changer in medical PSAs. The introduction of the MQ medical silicone resin, hailed as the “small-molecule wonder,” has become an indispensable functional factor in high-end medical PSA formulations, combining the unique properties, mechanical properties, and chemical resistance of silicone rubber with exceptional biocompatibility.
There are five aspects to introducing silicone resin in the medical field:
- What is MQ silicone resin?
- What is medical pressure-sensitive adhesive?
- What are the requirements for medical PSA?
- What are the advantages of MQ silicone resin in medical PSA applications?
- What are the industry applications of medical PSA?
- How to improve the performance of medical-grade silicone PSA?
1. What is MQ silicone resin?
MQ medical silicone resin is a highly branched siloxane polymer composed of M units (trimethylsiloxy, R3SiO1/2) and Q units (tetraoxysilyl, SiO4/2, derived from silicon dioxide). The MQ arrangement combines the flexibility of organic structural units with the durability and chemical stability of an inorganic siloxane (silica) network, resulting in a soft, rubber-like material with many benefits for medical applications:
Compact molecular structure/high cross-linking density (critical for mechanical properties and wear resistance)
Micron-sized particles with transparent to milky white appearance (suitable for transparent skin contact devices such as menstrual cups or baby bottle nipples)

Ultra-low surface energy with excellent hydrophobic and oleophobic qualities (resistant to water and many solvents)
Electrical insulation, aging resistance, and photo-oxidative stability
High physiological inertness: no irritation to skin or the body—considered safe and biocompatible
The MQ resin serves as a filler in the silicone rubber matrix, boosting stretch and adherence while reducing residue and irritation. In medical-grade silicone rubber, unnecessary fillers are avoided to ensure biocompatibility and safety.
These characteristics are why medical grade silicone, and more specifically MQ resin, is often the preferred elastomer in medical grade materials for devices like medical tapes, post-surgery dressings, medical grade tubing, and food contact items.
2. What is medical pressure-sensitive adhesive?
Medical pressure-sensitive adhesives (medical PSAs) can be broadly categorized into organic types (acrylic esters, SIS, PIB) and silicone-based types (medical grade silicone rubber). Medical grade silicones themselves are divided into categories based on their suitability for non-implantable, short-term, or long-term medical applications, helping determine the appropriate material according to regulatory standards and specific application requirements. Traditional PSAs using non-silicone materials sometimes compromise on critical features such as peelability or long-term adhesion. The addition of MQ medical silicone resin dramatically improves performance in medical-grade silicone PSA systems, resulting in medical adhesives that are exceptionally flexible, resistant to oxygen and solvents, suitable for all skin types, and reliably gentle for both infants and the elderly.
MQ resin is often included in medical applications as a tackifier, modifier, or adhesive balance regulator—a key additive in modern medical devices like medical tapes, band-aids, medical sensors, and patches. Today, MQ resin is widely regarded by manufacturers as essential to medical-grade PSA formulation and is featured in FDA-registered, FDA-approved products.

3. What are the requirements for medical PSA?
Medical PSAs are more than just “invisible support”—they are essential for materials used in medical applications like dressings, medical fixation tapes, sensors, and portable devices, such as computer chips for health monitoring, or for use on menstrual cups, baby bottle nipples, and food contact items.
Key requirements include:
Strong but gentle adhesion to the skin without causing damage, pain, or allergies
Ultra-long wear (e.g., 24-hour monitoring), even under extreme temperatures (body heat or cold exposure)
Non-toxic, non-irritating, hypoallergenic, and suitable for sensitive or damaged skin
Breathable yet waterproof—resistant to sweat, tears, or other secretions
Stable under sterilization, disinfection, or heat (thermal treatment); minimal residue for easier recovery
High flexibility for curved or moving body surfaces (joints, neck, etc.)
Leaves little or no residue—very important for hospital use and food contact.
Traditional acrylic and rubber PSAs often have drawbacks: excess tackiness, poor resistance to moisture, lacking reusability, or rapid aging. Pure medical grade silicone rubber PSAs alone may have insufficient “tack” or self-regulation. By integrating MQ organosilicon resin—blended with medical grade materials through a carefully controlled chemical process—these limitations can be overcome, improving adhesion, flexibility, durability, and skin-friendliness. Medical-grade silicone PSAs are manufactured under strict quality standards to ensure safety, purity, and suitability for medical use.
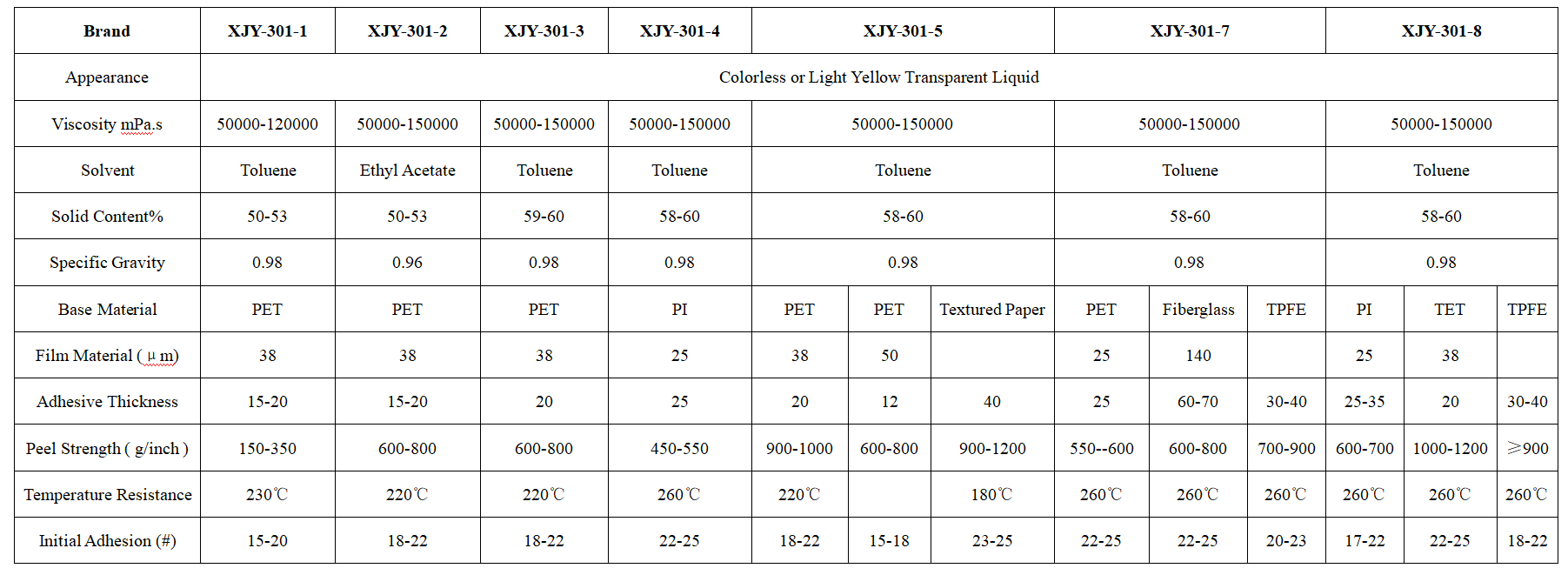
XJY-301 Silicone Pressure Sensitive Adhesive
It is made of silicone resin with a specific structure and high molecular weight polydimethylsiloxane with organic adhesive, which is applied in specific scenario conditions with a catalyst.
Compared with natural rubber, it is characterized by high-temperature resistance, high stability, good electrical insulation, durable performance(original shape can not change), good transparency, high peel adhesion, etc.
Its high performance has a wide range of applications in industrial product processing, electronic processing, optical materials, health care (protective films), and other fields.
4. What are the advantages of MQ silicone resin in medical PSA applications?
(1) Optimal balance of adhesion and peelability
MQ resin enables precise molecular regulation by adjusting the M/Q ratio for medical applications, allowing for a combination of rigidity and flexibility in the adhesive. This maintains adhesion while making removal easy, soft, and less damaging to the body. The MQ resin serves as a filler in the silicone rubber matrix, boosting stretch and adherence while reducing residue and irritation. Its unique properties allow it to be used even in liquid silicone rubber base form for custom molding and extrusion. When medical-grade silicone is stretched, it should not change color or reveal fillers, which demonstrates its high purity and suitability for medical applications.

(2) Excellent breathability and skin compatibility
The microstructure of MQ resin, akin to a silica mesh, permits both oxygen and water vapor exchange, preventing wear, heat buildup, or irritation. This contributes to improved comfort during extended wear, making MQ resin the preferred choice for biomedical applications.
(3) Exceptional resistance to aging, temperature, and humidity
The inherent stability of the siloxane network provides resistance to extreme temperatures, light, solvents, and sweat, while remaining non-toxic, biocompatible, and non-irritating—all essential for medical grade and FDA registered applications.
(4) Perfect surface performance, no residue left behind
MQ resin ensures a dense, smooth finish for adhesives, reducing wear and preventing the sticking of fibers or other materials to wound sites. Devices can continuously and reliably adhere to the skin and are easily removed without damage or marks.
(5) Enables special “smart adhesion” and product innovation
MQ resin’s unique compounding allows PSAs to be engineered for flexibility, softness, and elasticity, which can flex and stretch with the body at joints or on the neck, without peeling off. This is vital for devices such as wearable healthcare monitors. It also enables manufacturers to mold transparent, color-custom, nano/micro-structure adhesives, serving today's drive for cost-effective, personalized, and widely-used medical solutions.
5. What are the industry applications of medical PSA?
MQ medical silicone resin is used throughout the market in medical grade silicone applications, from high-end band-aids, device patches, menstrual cups, food contact products, to beauty repair patches and sports medical tapes. Some notable examples:
Band-aids and dressings: Secure adhesion, painless removal, suitable for all skin types and ages; medical grade silicone rubber ensures both durability and gentleness.
ECG/EEG sensor patches: Rely on MQ resin’s flexibility and adaptability for long wear, consistent signal transmission, and repeated use without residue.
Wound care fixation tape: MQ resin’s elasticity and tear resistance are essential for surgical tapes and drainage tubes, especially waterproof or antibacterial types.
Wearable monitors/glucose/insulin pumps: Ultra-long-term adherence to the body, high tolerance to extreme temperatures, and biocompatibility are a must—MQ resin delivers.
Children/elderly/special population tapes: Hypoallergenic, FDA registered formulations using medical grade silicone are vital to prevent skin damage or irritation.
Cosmetic/beauty patches: Transparent, high stability, and washability for medical beauty and scar treatment patches.
Sports/emergency tapes: Rapid cure, “second-adhesion,” stretch, and resistance in sweating or outdoor scenarios.
For example, compared to plastic, medical-grade silicone offers superior durability and biocompatibility, making it a preferred choice for sensitive medical applications. Medical grade silicone is available in different grades and categories, each designed for specific uses such as implants, wound care, or devices where contact with bodily fluids may occur. The main difference between medical-grade silicone and conventional silicone lies in the higher purity, strict regulatory standards, and enhanced safety required for medical use. Silicone is derived from sand, which contains silicon dioxide, through a chemical process that transforms it into a versatile synthetic material. Products like the menstrual cup rely on medical-grade silicone because they must be safe and non-reactive when contact with human tissue and fluids occurs, highlighting the importance of biocompatibility and safety.

6. How to improve the performance of medical-grade silicone PSA?
Medical-grade silicone PSAs need to balance just-right adhesion and clean removal. The MQ organosilicon resin, as a medical grade filler for liquid silicone rubber or medical grade silicone rubber, is key to achieving high-performance, flexible, biocompatible, and cost-effective results. Using MQ resin means leveraging the best of both mechanical and physical properties of medical-grade silicone and silicon for next-generation devices and biomedical applications.
XJY Silicones is one of China's leading manufacturers of medical-grade silicone, MQ resin, and VMQ resin, with over 30 years of expertise in chemical process development and manufacture. Our medical-grade materials are FDA-approved, widely used, and produced for a spectrum of healthcare and medical applications, supporting custom engineering needs worldwide.
